STATE OF AFFAIRS: OREGON ATTORNEY GENERAL TAKES ON BIG PHARMA
Rosenblum Settles with Pharmaceutical Company Insys Over Unlawful Promotion
 SALEM, Oregon (April 21, 2016) – Oregon Attorney General Ellen Rosenblum recently reached a $1.1 million settlement with Insys, a company that manufactures Schedule II opioid drug Subsys, to resolve allegations that the powerful drug approved by the Food and Drug Administration (FDA) to treat cancer pain was marketed in the state for off-label uses like non-cancer neck and back pain.
SALEM, Oregon (April 21, 2016) – Oregon Attorney General Ellen Rosenblum recently reached a $1.1 million settlement with Insys, a company that manufactures Schedule II opioid drug Subsys, to resolve allegations that the powerful drug approved by the Food and Drug Administration (FDA) to treat cancer pain was marketed in the state for off-label uses like non-cancer neck and back pain.  “Sybsys is a very powerful narcotic that has been approved for only a very specific and narrow use,” said Attorney General Rosenblum. “Schedule II drugs have a very high potential for use and addiction, and it is unconscionable that a company would promote such a powerful drug for off-label uses as well as misrepresent to doctors the benefit of the drug.” Insys released a statement after the settlement, saying it is “committed to developing products for the supportive care of patients through the use of its drug delivery technologies. Insys takes patient safety very seriously and is committed to working with the healthcare community, including health care providers, or HCPs, payers, pharmacies, and most importantly, patients to help ensure the proper prescribing and use of its products.”
“Sybsys is a very powerful narcotic that has been approved for only a very specific and narrow use,” said Attorney General Rosenblum. “Schedule II drugs have a very high potential for use and addiction, and it is unconscionable that a company would promote such a powerful drug for off-label uses as well as misrepresent to doctors the benefit of the drug.” Insys released a statement after the settlement, saying it is “committed to developing products for the supportive care of patients through the use of its drug delivery technologies. Insys takes patient safety very seriously and is committed to working with the healthcare community, including health care providers, or HCPs, payers, pharmacies, and most importantly, patients to help ensure the proper prescribing and use of its products.” 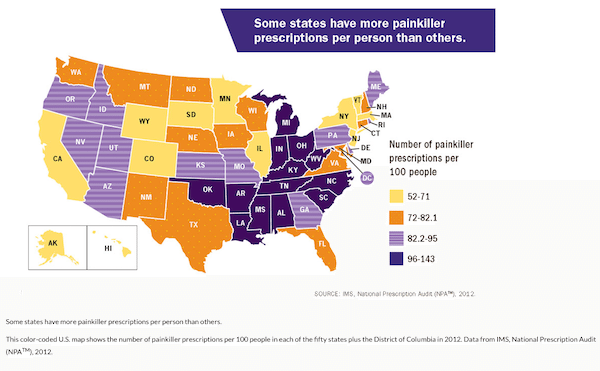 The Assurance of Voluntary Compliance (AVC) claims that the company provided improper financial incentives to some doctors to increase Subsys prescriptions, targeted doctors for aggressive promotion of the drug when the doctor was not qualified to prescribe it, and promoted it for treatment of mild pain. Oregon is the first state to settle with the drug company for the allegations. Under the conditions of the settlement, Insys must follow federal anti-kickback laws and not market Subsys in the state for mild pain of any kind unless the FDA approves that claim. Insys also has to pay Oregon $533,000, and an additional $567,000 to nonprofits to prevent opioid use. Sales for Subsys in the state were upwards of $500,000.
The Assurance of Voluntary Compliance (AVC) claims that the company provided improper financial incentives to some doctors to increase Subsys prescriptions, targeted doctors for aggressive promotion of the drug when the doctor was not qualified to prescribe it, and promoted it for treatment of mild pain. Oregon is the first state to settle with the drug company for the allegations. Under the conditions of the settlement, Insys must follow federal anti-kickback laws and not market Subsys in the state for mild pain of any kind unless the FDA approves that claim. Insys also has to pay Oregon $533,000, and an additional $567,000 to nonprofits to prevent opioid use. Sales for Subsys in the state were upwards of $500,000. 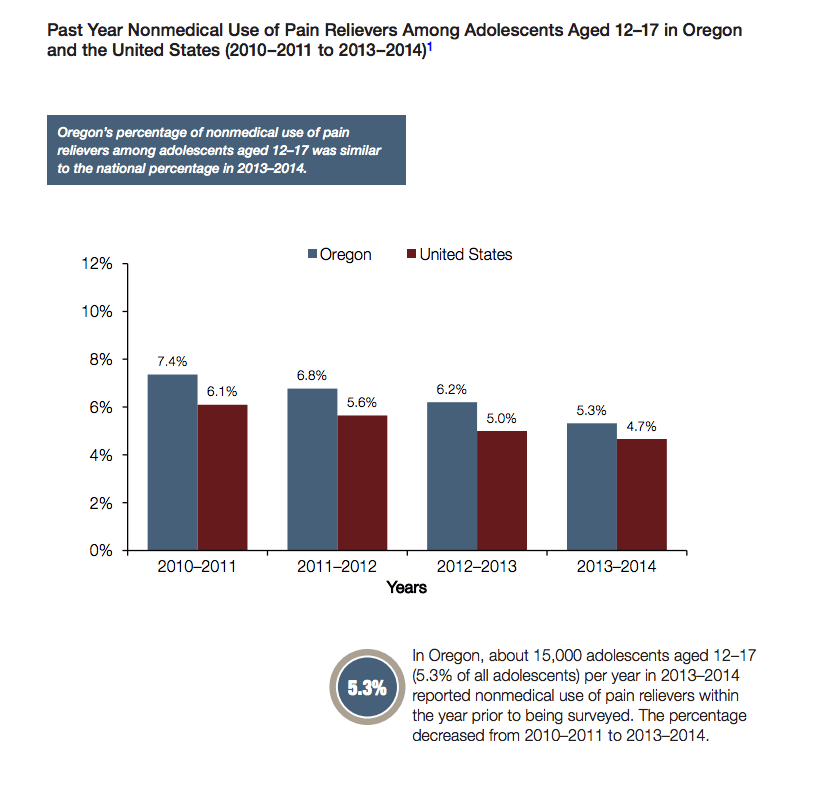 Attorney General Rosenblum also announced a $2.1 million grant to help combat opioid use, funded by the $28 million Neurontin settlement from 2004. Oregon is 20th in the nation for opioid prescriptions, averaging 89 prescriptions per 100 people in the state. Top spots in that category belong to Alabama, West Virginia and Tennessee. Oregon’s percentages of illicit drug use and alcohol binge drinking among adolescents were both higher than the national percentage last year, and opioid use was about the same. Perhaps other states will take a step in holding pharmaceutical companies accountable, as we join New Zealand as the only two countries in which they are allowed to advertise on television, and are one of few countries that do not charge a percentage of the profits to fund addiction treatment and prescription take backs – like the one that will be held at Vertava Health headquarters Saturday, April 29 in conjunction with the Brentwood, Tennessee Police Department and Williamson County Anti-Drug Coalition.
Attorney General Rosenblum also announced a $2.1 million grant to help combat opioid use, funded by the $28 million Neurontin settlement from 2004. Oregon is 20th in the nation for opioid prescriptions, averaging 89 prescriptions per 100 people in the state. Top spots in that category belong to Alabama, West Virginia and Tennessee. Oregon’s percentages of illicit drug use and alcohol binge drinking among adolescents were both higher than the national percentage last year, and opioid use was about the same. Perhaps other states will take a step in holding pharmaceutical companies accountable, as we join New Zealand as the only two countries in which they are allowed to advertise on television, and are one of few countries that do not charge a percentage of the profits to fund addiction treatment and prescription take backs – like the one that will be held at Vertava Health headquarters Saturday, April 29 in conjunction with the Brentwood, Tennessee Police Department and Williamson County Anti-Drug Coalition.